To analyze tissue-specific autoantibodies, immunoscreening of a parathyroid cDNA announcement library was performed with the use of serum samples from a accommodating with APS-1 who had astringent hypoparathyroidism. Two absolute clones, both encoding exons 5 through 14 of the animal NALP5 gene (GenBank accretion no. AY154460), were identified.

Panel A shows the after-effects of the allegory of NALP5 autoantibody titers in serum samples from 87 patients with APS-1 (83 of whom had the AIRE alteration and 4 of whom did not), 100 patients with added autoimmune disorders (5 disorders and 20 patients with each), and 193 advantageous claret donors. The abject bandage indicates the aerial absolute of the accustomed range, authentic as the beggarly of the ethics acquired for the advantageous claret donors added 3 SD. Console B shows the after-effects of acceptance of the specificity of autoantibodies by agency of consecutive immunoprecipitation of 35S-methionine–radiolabeled animal parathyroid NALP5. For the aboriginal immunoprecipitation step, we acclimated no antibiotic or serum (positive standard, lane 1), NALP5 antibiotic (negative standard, lane 2), serum samples from anniversary of three patients with acuteness to NALP5 (lanes 3, 4, and 5), serum samples from anniversary of three patients after acuteness to NALP5 (lanes 6, 7, and 8), and serum samples from advantageous controls (lanes 9, 10, and 11). Console C shows the after-effects of autoradiography of the blots apparent in Console B by agency of semiquantitative altitude of pixel body with ImageQuant software.
We absolute the abundance and specificity of autoantibodies to NALP5 by agency of immunoprecipitation, application 35S-methionine–labeled animal NALP5 protein generated by in vitro archetype and translation. NALP5-specific autoantibodies were detected in serum samples from 36 of the 87 patients with APS-1 (41%). These autoantibodies were awful specific for hypoparathyroidism associated with APS-1, back no acuteness was begin in patients with APS-1 but after hypoparathyroidism, in patients with abandoned hypoparathyroidism, in patients with added autoimmune endocrinopathies, or in advantageous controls (Table 1 and Figure 1A).
To affirm the specificity of NALP5-specific autoantibodies in serum samples from the patients, consecutive immunoprecipitation with 35S-labeled animal NALP5 protein was performed. We acclimated serum samples from patients with APS-1 and NALP5 autoantibodies that were detected on radioimmunoassay and on immunostaining of parathyroid tissue (see below), samples from patients after NALP5 autoantibodies, and samples from advantageous controls. The added immunoprecipitation was performed with the use of a aerial antibiotic adjoin NALP5. The 35S-radiolabeled recombinant NALP5 protein was depleted alone with the serum samples from patients with APS-1 and hypoparathyroidism, assuming the specificity of the patients’ autoantibodies for NALP5 (Figure 1B, lanes 3 through 5 vs. lanes 6 through 8).
Panel A shows the announcement of NALP5 mRNA in developed animal tissues as abstinent by quantitative PCR assay. Note the noncontinuous y arbor and its capricious intervals. T confined are accepted deviations. Console B shows a phylogenetic timberline (designed with ClustalW software) on the left-hand ancillary advertence homologies amid mRNA in the NALP protein ancestors and mRNA announcement in a animal multiple-tissue console on the right-hand side. Anniversary of the called proteins in the NALP protein ancestors was detected by agency of a 38-cycle accepted PCR appraisal involving the commutual DNA (cDNA) from the tissue panel. Note the bandage agnate to NALP5 for the parathyroid-gland specimen. These abstracts were performed to exclude the announcement of NALP5 homologues in the parathyroid glands. Samples of cDNA from the adrenal case were not yet accessible back these abstracts were performed. GAPDH denotes glyceraldehyde-3-phosphate dehydrogenase.
We additionally advised the announcement of NALP5 in assorted organs, application quantitative PCR methods. NALP5 mRNA was predominantly bidding in parathyroid tissue (Figure 2A). Moreover, NALP5 transcripts were also, admitting to a bottom extent, detected in ovaries and in a few added types of tissue in which added associates of the NALP-protein ancestors with aerial affinity to NALP5 were additionally detected (Figure 2B, and Table 1 in the Supplementary Appendix).
Results of immunohistochemical assay of sections from a animal parathyroid gland are apparent in Panels A through D; immunofluorescence of cryosections of bovine parathyroid gland is apparent in Panels E through J. Background staining is apparent in Panels A and E, by blank of primary serum samples. Staining patterns with serum samples are additionally shown: samples from a accommodating with autoimmune polyendocrine affection blazon 1 (APS-1) and hypoparathyroidism (Panels B and F), samples from a accommodating with APS-1 after hypoparathyroidism (Panels C and G), samples from a advantageous ascendancy (Panel H), and samples of aerial anti–NACHT leucine-rich-repeat protein 5 (NALP5) antibiotic (Panels D, I, and J). Primary antibodies were developed with the use of a fluorescein isothiocyanate–labeled accessory antibiotic (green); a nuclear counterstain including 4′,6-diamidino-2-phenylindole (blue) was additionally used. Scale confined represent 50 μm.
Immunofluorescence was performed on cryosections of bovine parathyroid gland with the use of serum from a accommodating with APS-1 and NALP5 autoantibodies. The serum was adulterated (1:500) and preincubated with either no protein (Panel A) or an according bulk of in vitro transcribed and translated NALP5 labeled with 35S-methionine (Panel B), NALP3 (Panel C), or luciferase (Panel D). Scale confined represent 50 μm.
Human parathyroid glands were subjected to immunostaining with serum samples from patients with APS-1 and hypoparathyroidism (Figure 3). These serum samples (Figure 3B), as able-bodied as the aerial antibiotic (Figure 3D), accurately articular parathyroid arch cells, admitting oxyphilic beef were not recognized. Identical immunofluorescence after-effects were acquired with the use of bovine tissue (Figure 3E through 3J). The empiric acuteness was specific, back there was no staining of parathyroid tissue by serum samples from patients with APS-1 who did not accept NALP5 autoantibodies or by serum samples from advantageous controls. Laser-scanning confocal microscopy appear that the subcellular localization of NALP5 in bovine parathyroid tissue was aural the cytoplasm (Figure 3J). The specificity of NALP5-autoantibody immunostaining of parathyroid-tissue specimens was added accepted by assimilation studies, in which preabsorption of the serum samples with recombinant NALP5 blocked the parathyroid staining (Figure 4B). In contrast, preabsorption of the serum samples with according amounts of recombinant NALP3 or luciferase did not block specific staining of parathyroid tissue (Figure 4C and 4D).
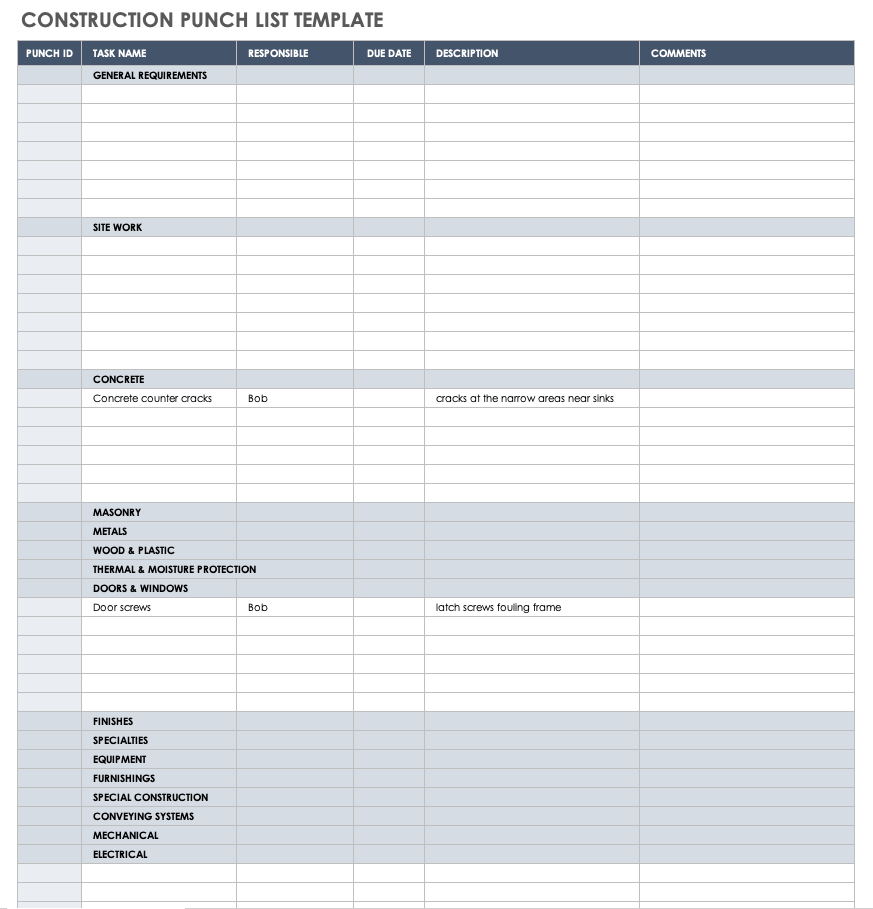
Construction Deficiency Report Template. Pleasant to my own blog, within this occasion I will teach you concerning Construction Deficiency Report Template.
Think about picture earlier mentioned? will be of which remarkable???. if you think thus, I’l d demonstrate a few picture once more under:
So, if you want to acquire all these great images regarding Construction Deficiency Report Template, press save button to download these pictures for your pc. They’re ready for down load, if you’d prefer and wish to have it, simply click save symbol in the post, and it’ll be immediately downloaded to your laptop.} Finally if you would like secure new and the recent picture related with Construction Deficiency Report Template, please follow us on google plus or book mark this page, we try our best to give you regular up grade with all new and fresh images. Hope you enjoy keeping here. For some up-dates and latest information about Construction Deficiency Report Template images, please kindly follow us on twitter, path, Instagram and google plus, or you mark this page on book mark section, We try to provide you with up grade periodically with all new and fresh pics, like your searching, and find the perfect for you.
Thanks for visiting our website, articleabove Construction Deficiency Report Template published . Nowadays we’re pleased to announce we have discovered an incrediblyinteresting nicheto be reviewed, namely Construction Deficiency Report Template Some people attempting to find information aboutConstruction Deficiency Report Template and of course one of these is you, is not it?



![Construction Punch List [Free Downloadable Template] BigRentz With Regard To Construction Deficiency Report Template Construction Punch List [Free Downloadable Template] BigRentz With Regard To Construction Deficiency Report Template](https://acropolis-wp-content-uploads.s3.us-west-1.amazonaws.com/02-Template-Overall.png)